 Zinc and lead are important industrial metals also known as “base metals.” But what types of products are made from zinc and lead, and why are they so important to everyday life.
Zinc and lead are important industrial metals also known as “base metals.” But what types of products are made from zinc and lead, and why are they so important to everyday life.
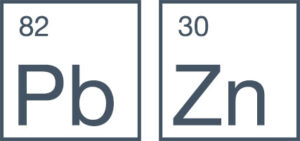
Zinc is a chemical element with the symbol Zn and atomic number 30. It is a brittle transition metal at room temperature but malleable at higher temperatures. Zinc is also known for its anti-corrosive properties.
Zinc is an important metal with many applications. It is primarily used for galvanization of cars, so they do not rust and for steel used within infrastructure since it provides superior protection against corrosion compared to iron or steel alone.
Zinc is also used in alloys such as brass (an alloy composed mainly of copper and zinc), which has a wide range of uses. It’s used in automobile parts such as batteries, brake pads, pistons, and cylinder liners.
Zinc oxide can be found in cosmetics like sunscreen lotions, because it helps absorb ultraviolet rays from the sun. Finally, zinc is often added to food products like breakfast cereals to help maintain healthy levels of essential minerals like iron in our bodies.
Zinc sulphate is used in the agricultural industry to rejuvenate soil and increase vegetation growth rates.
Lastly Zinc carbonate is a supplement that is used to boost immunity.
Lead is a chemical element with the symbol Pb and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable and has a relatively low melting point.
Lead has been used throughout history for a variety of purposes. It was used in ancient Rome to make water pipes and as a component of solder. Lead was also used in medieval Europe to make stained glass windows.
Today, it is primarily used for making batteries, radiation shields, bullets, pipes, bearing alloys, solder, soldered joints, weights for balancing aircraft tires, counterweights for elevators and escalators, cable sheathing material to protect against corrosion and electrical components such as cables and wires, among other uses.
Lead is also commonly used in paints due to its excellent corrosion resistance.

